Biological medicines: what are they?
Stemming from biotechnologies, biological medicines are also called biomedicines or biotherapies. They are generally produced from living cells using recombinant DNA technologies, or genetically modified cells. These techniques help to develop vaccinations, hormones, transformed viruses, modified cells or plants. Biomedicines help to treat serious illnesses: cancers, autoimmune diseases, diabetes, etc.
These medicines are affected by the challenges of the cold chain. Out of the world’s 10 best-selling pharmaceutical products in 2014, 5 medicines must be stored at a specific temperature range (4 between +2°C and +8°C, 1 between +15°C and +25°C) and these concern biomedicines.
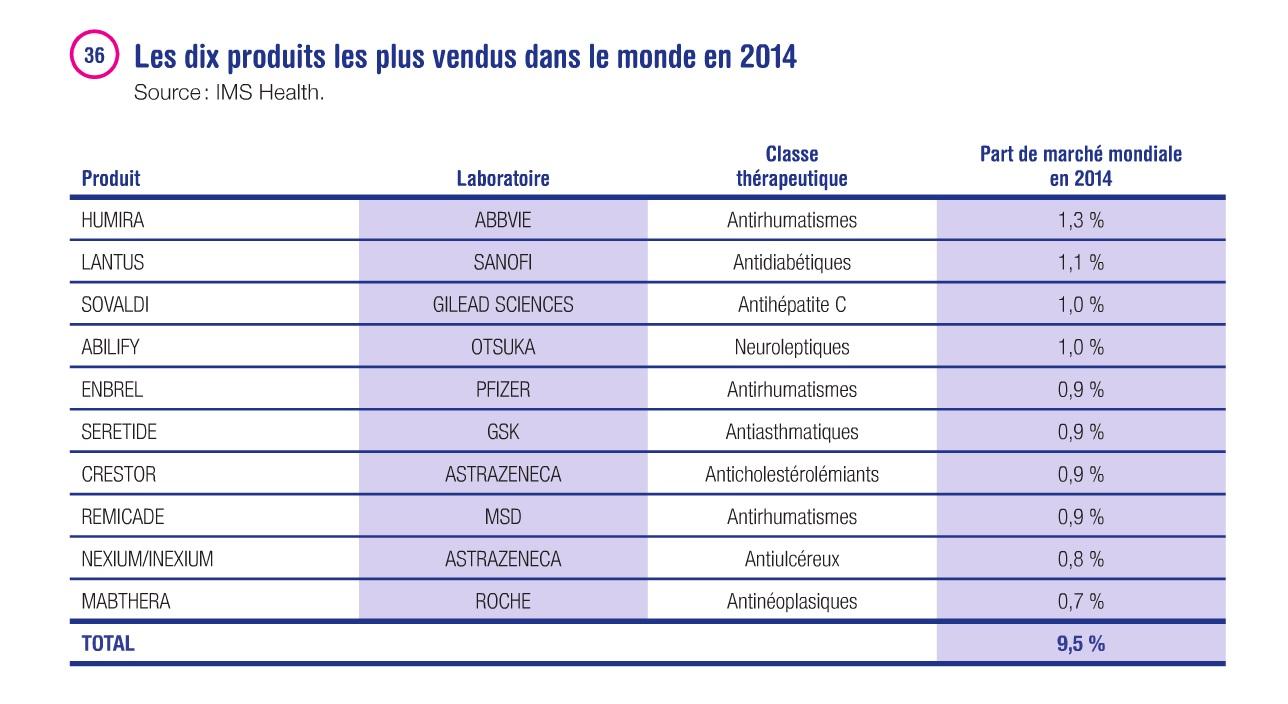
Top 10 of the world’s best-selling medicines in 2014 (source IMS Health)
Recent healthcare innovations depend largely on the development of biological medicines
Today, 80% of new molecules on the market stem from biotechnologies. In 2005, they only represented 20 to 30% of the market, according to the report “The place of biotechnologies in France and Europe” by the MP Jean-Yves Le Déaut, President of OPECST (Parliamentary Office for Evaluation of Scientific and Technological Options).
In 2014, out of the world’s 20 best-selling pharmaceutical products, 11 were biological medicines, according to IMS Health - which also predicted an increase in the biomedicine market. It is estimated to reach 250 billion dollars by 2020.
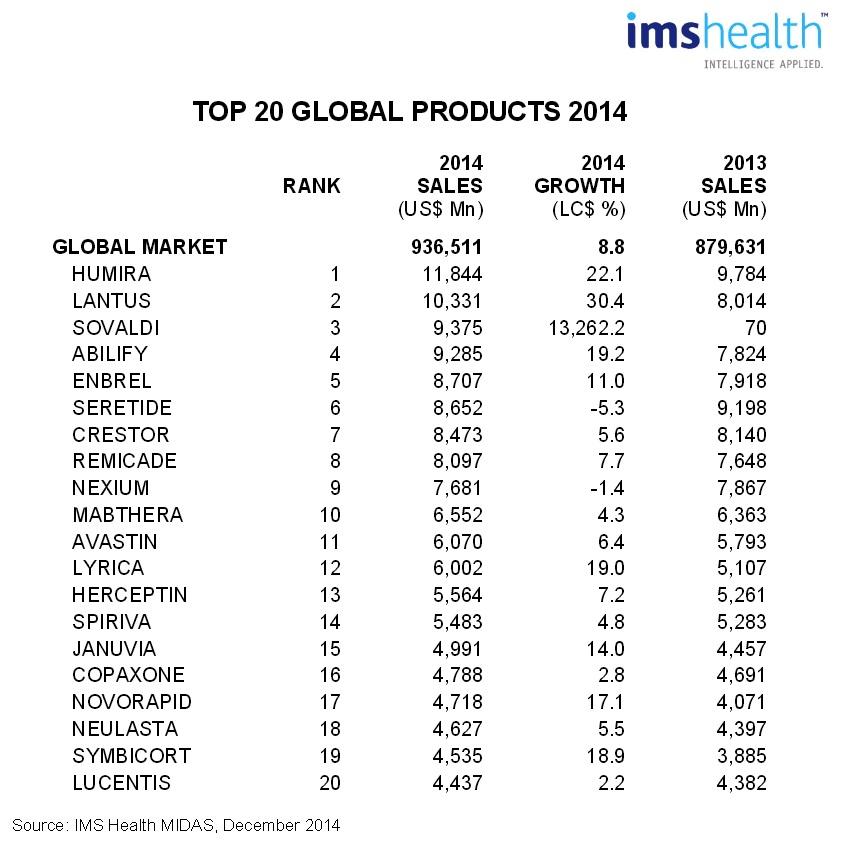
Top 20 of the world’s best-selling pharmaceutical products in 2014
In order to develop new treatments, pharmaceutical companies are turning towards open innovation and are calling on cutting-edge start-ups capable of developing precision medicines. Oncology, for example, will be a key area for orphan medicines, 40% of which stem from biotechnologies. These therapeutic approaches make up one of the foundations of the concept of personalised medicine.
Biosimilars: a market opportunity

Some patents for biological medicines will come into the public domain over the next 5 years. In concrete terms, 9 biomedicines which themselves alone generate 40% of the current global market for biological medicines will soon see (or have recently seen) their patent expire. This paves the way for the marketing of biosimilars and is a very attractive opportunity for manufacturers (this market will represent between 11 and 25 billion dollars in 2020). Pharmaceutical laboratories are already developing biosimilars – modelled on generic medicines, alternatives to chemical medicines.
Moreover, health organisations are exerting pressure to control healthcare spending: the cost of a biological medicine is higher than that of other medicines. A biosimilar medicine is on average 20 to 30% cheaper than a reference biological medicine and would therefore make savings.
Lastly, biosimilars would allow developing countries easier access to adapted treatments, particularly for conditions which require excessively costly and inaccessible care for patients who do not benefit from social security.
The cold chain more topical than ever
With the development of biological medicines and biosimilars, compliance with the cold chain is going to become a recurring theme in the years to come.
Everybody involved in healthcare is affected by the storage of these medicines: pharmaceutical companies, logisticians, distributors, clinics and hospitals, pharmacies, and of course the patients. Everyone must be aware of the appropriate actions and good practices they must follow to ensure the effectiveness of the treatments.
