News
Thematic studies
UN3373 insulated packaging to secure the shipment of biological samples
Thousands of samples of human or animal origin are collected and sent around the world every day by pharmaceutical companies, nursing homes, diagnostic laboratories, research centres and others. They are collected as part of medical tests, clinical trials, surveillance studies and various other tests. The results of clinical trials are crucial for market authorisation dossiers submitted by pharmaceutical companies to the relevant authorities. Samples must be transported in accordance with IATA regulations for the air transport of dangerous goods and with the European agreement concerning the International Carriage of Dangerous Goods by Road (ADR). Temperature-sensitive samples must for their part be transported in full compliance with the cold chain, on which their effectiveness depends. Depending on their stability, they are transported between +2 and + 8°C (or between +2 and +4°C) or below -18°C. To guarantee the cold chain, laboratories, depositories and CRO units use cooling packaging. For home treatment, qualified insulated bags are used to transport heat sensitive products. Samples (blood, skin, etc.) are transported in UN 3373 insulated packaging, from the collection site (hospitals or laboratories) to analysis laboratories.
UN 3373 packaging: Transport of class B biological substances
The ADR and IATA regulations for air and road transport of infectious substances which may contain pathogens are based on UN recommendations. Category A infectious substances, regulated by UN 2814 and UN 2900, are those which can cause permanent disability or fatal illness in humans or animals. Category B infectious substances are those which are not classified as category A. It is regulated by UN 3373, and its official transport designation is « BIOLOGICAL SUBSTANCE, CATEGORY B ».
UN 3373 packaging consists of three layers:
- Leak-proof primary packaging (container) that contains the substance ;
- Secondary packaging, which is resistant and leak-proof, protecting one or more primary containers wrapped in an absorbent material enough to absorb all liquid in case of leakage.
- Outer packaging that protects its contents and which guarantees the cold chain if necessary.
UN 3373 packaging must be of good quality, strong enough to withstand the shocks and loadings normally encountered throughout the logistics circuits (transport, handling, manual or mechanical handling...). Packaging must be constructed and closed so as to prevent any loss of contents that might be caused under normal conditions of transport, by vibration, or by changes in temperature, humidity or pressure.
Depending on the material transported liquid or solid, the primary receptacle and secondary packaging must meet leakproofness mechanical strength requirements.
For liquid substances:
- The primary receptacle(s) must be leakproof ;
- The secondary packaging must be leakproof ;
- If multiple fragile primary receptacles are placed in a single secondary packaging, they must be either individually wrapped or separated to prevent contact between them ;
- Absorbent material must be placed between the primary receptacle and the secondary packaging. The absorbent material must be in sufficient quantity to absorb the entire contents of the primary receptacle(s) so that any release of the liquid substance will not compromise the integrity of the cushioning material or of the outer packaging ;
- The primary receptacle or the secondary packaging must be capable of withstanding, without leakage, an internal pressure of 95 kPa (0.95 bar).
In addition to these requirements the IATA adds the following conditions:
- The primary receptacle(s) must not contain more than 1 L ;
- The primary receptacle or the secondary packaging must be capable of withstanding a pressure of 95 kPa in the range of -40°C to 55°C (-40°F to 130°F).
- The outer packaging must not contain more than 4 L. This quantity excludes the cold source used to keep specimens in the required temperature range.
For solid substances:
- The primary receptacle(s) must be siftproof ;
- The secondary packaging must be siftproof ;
- If multiple fragile primary receptacles are placed in a single secondary packaging, they must be either individually wrapped or separated to prevent contact between them ;
- If there is any doubt as to whether or not residual liquid may be present in the primary receptacle during transport then a packaging suitable for liquids, including absorbent materials, must be used.
In addition to these requirements the IATA adds the following conditions:
- and must not exceed the outer packaging weight limit ;
- except for packages containing body parts, organs or whole bodies, the outer packaging must not contain more than 4 kg. This quantity excludes the cold source used to keep specimens in the required temperature range.
The constraints to be respected so that the cold chain packaging complies with regulations
Cold chain packaging for clinical specimens must comply with IATA regulations. The outer packaging should respect constraints about dimensions, design and about resistance. The packaging must be properly marked, labelled and accompanied by shipping documents.
- Dimension: At least one surface of the outer packaging must have a minimum dimension of 100 mm × 100 mm.
- Design: To transport the marking below must appear on an external surface of the pack on a bottom surface of contrasting color and be clearly visible and legible. The marking shall be square in shape and disposed at an angle of 45° (diamond shaped), each side measuring at least 50mm long. The line width must be at least 2 mm and the letters and numbers must be at least 6mm high.
- Mandatory mention: The proper shipping name should be "Biological substance category B" are printed in letters not less than 6mm high. It must appear on the pack next to the diamond shape.
- Resistance: Once the loan package must be able to pass the drop test specified in the IATA - Chapter 6.5.1.1 the height of the fall must not be less than 1.2m. After the series of falls indicated, there shall be no leakage from a primary receptacle, which must remain protected by absorbent material, if required in the secondary packaging.
Refrigerated or frozen specimens
When dry ice or liquid nitrogen is used to as a cold source keep specimens in a required temperature range, all applicable requirements of these regulations must be met. In this case, the cold source must be placed outside the secondary packaging or in the outer packaging.
Interior supports must be provided to secure the secondary packaging in the original position after the ice or dry ice has dissipated.
If dry ice is used, the packaging must be designed and constructed to permit the release of carbon dioxide gas to prevent a build-up of pressure that could rupture the packaging.
The primary receptacle and the secondary packaging must maintain their integrity at the temperature of the refrigerant used as well as the temperatures and the pressures, which could result during transport.
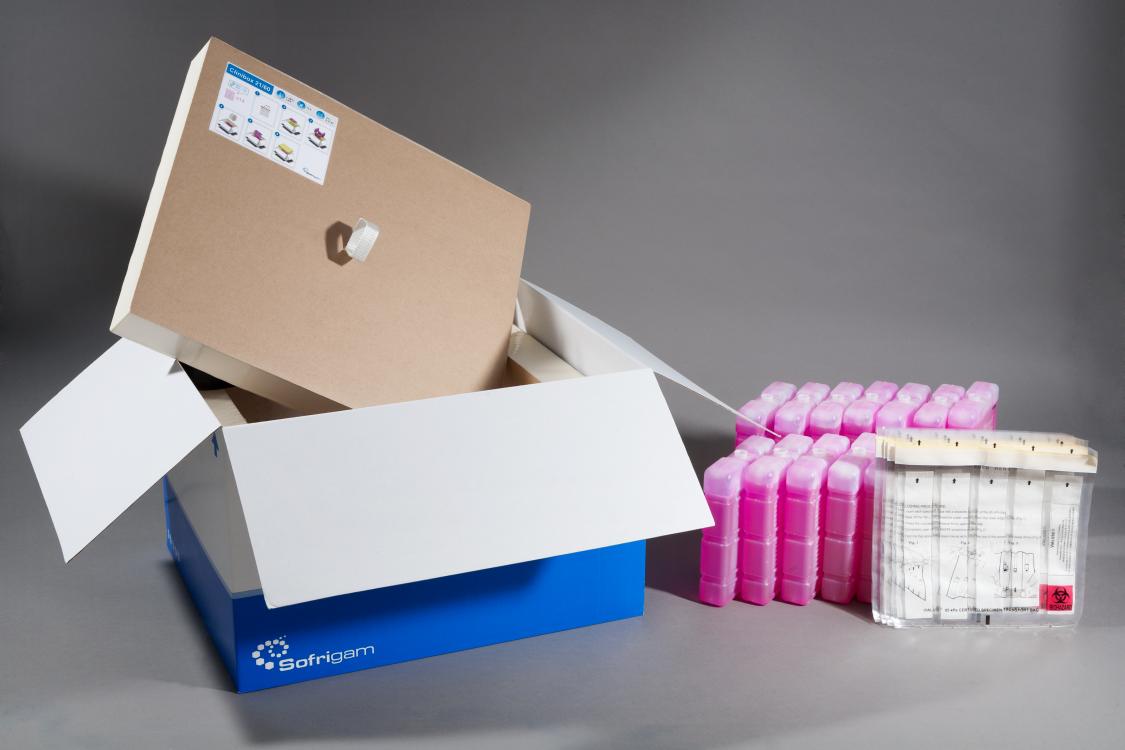
Clinibox°: insulated packaging dedicated to clinical trials
Sofrigam has developed a range of UN 3373 packaging dedicated to clinical trials. Clinibox° ensures the safety of temperature-controlled transport of clinical trials (clinical samples, diagnostic specimens or category B biological substances). It guarantees both compliance with regulations for the transport of dangerous goods (IATA and ADR) and the respect of the cold chain.
These UN 3373 transport kits consist of VIAL 650 pouches (secondary packaging) and a refrigerated box (outer packaging). The VIAL 650 pouch is 95 kPa certified, by an IATA approved laboratory. It is divided into several separate compartments pre-equipped with the absorbent material. Tubes are directly placed in these compartments, thus complying with the requirements of separation and protection against spillage.
The insulated boxes are made of rigid polyurethane panels and refrigerated, according to needs, by eutectic gel packs or dry ice. They are tested and qualified by our partner Ater Métrologie laboratory, according to ISTA 7E and NF S 99-700 standards for thermal performance. They have also successfully passed drop tests from a minimum height of 1.2 m.
Clinibox° packaging range covers temperatures between +2 and +8 °C or below -18 °C, and durations from 24 up to 96 hours. It may be expanded for other specific needs: other volumes, other durations and other temperature ranges.
Clinibox° insulated packaging range
Cold chain packaging specifically developed to transport category B biological products.