Thematic studies
News
Case studies
Thermochemical sorption: a solution for temperature-controlled transport
Thermochemical storage based on a solid-gas chemical sorption process is used in temperature-controlled insulated transport containers. The principle is based on the coupling, via a gas phase, of a liquid/gas change of state of a natural fluid (ammonia) and a reversible reaction between the latter and a reactive solid. It thus allows a deferred and regulated production of cold offering autonomy to the container (roll) during transport. This article presents the qualification of such a temperature-controlled refrigerated container allowing the transport of products between 0°C and +4°C. Depending on the external temperature profile, the autonomy of the container can vary from 16 to 72 hours.
 Introduction
Introduction
Thermal energy is used in all sectors of economic life and represents the largest share of final energy consumption. Thermal storage compensates for the time lag between energy demand and the availability of the resource, improves energy efficiency and reduces greenhouse gas emissions. It also offers an effective cooling capacity for temperature-controlled transport and reduces the logistical costs required to maintain the cold chain.
Thermal storage may be envisaged using phase-change materials (PCM) or based on heat sorption of a thermochemical material (TCM). This concept has very promising potential: high-capacity heat or cold storage for long periods practically without any loss.
Thermochemical storage
Thermochemical storage exploits the heat sorption carried out in the reversible physical-chemical processes of exothermic absorption and endothermic desorption of a gas (G) on a sorbent medium (S): (SG) + heat D S+G. Depending on the nature of the sorbent medium (liquid or solid), the following can be exploited:
- heat from dilution of gas in a liquid solution (binary NH3/H2O or saline LiBr/H2O),
- heat from crystallisation / dissolution of a salt in a saturated solution (hydrates)
- heat from adsorption of a gas (H2O, NH3, MeOH) on the surface of a microporous, adsorbent material , such as activated carbon, zeolite or silica gel,
- heat from a reaction between a gas (H2O, NH3, CO2, H2) and a solid reagent (hydrates, hydroxides, ammoniacates, carbonates or metal hydrides).
The implementation of thermochemical storage involves the management of both reversible physical-chemical processes coupled by mass via the gaseous phase: the process of absorption/desorption of the gas by the sorbent medium and evaporation/condensation of this gas. This process takes place in two tanks (a condenser/evaporator and a reactor) each equipped with heat exchangers, connected to one another by a valve.
During the storage phase, the high temperature heat supply allows endothermic desorption of the gas from the sorbent. The gas released is then liquefied in a condenser and stored at room temperature (Fig. 1a).
When the sorbent is sufficiently discharged into a gas, the valve is closed, separating the two constituents (Fig. 1b). Thermal energy is then stored in the form of chemical potential in separate constituents, indefinitely in time with no loss of energy.
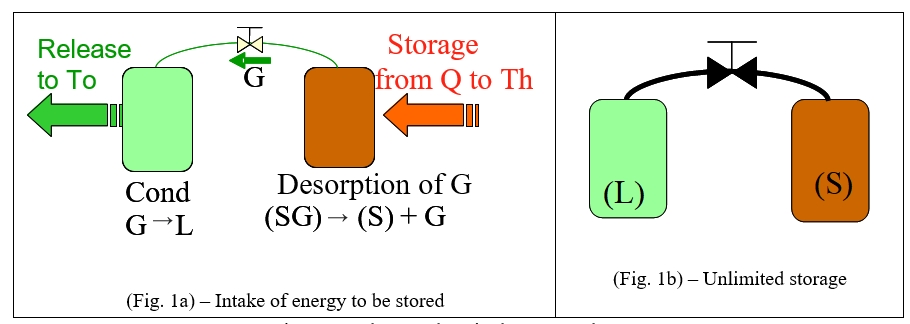
Figure 1: Thermochemical storage phase
The retrieval phase consists of making the two separate constituents react again: heat supply to the evaporator (cold production) produces a gas through evaporation which is exothermically reabsorbed by the sorbent. Depending on the operating conditions (evaporation temperature, room temperature), the nature of the sorbent material (solid, liquid, variance of the system) and the working fluid (H2O, NH3, CO2, H2,…) used, there are three possible applications during the retrieval phase (Fig. 2):
- production of cold with the evaporator (Fig. 2a),
- recovery of heat in Th previously stored in the reactor also in Th (Fig. 2b),
- production of heat in the reactor at a higher temperature than that of the heat stored as a result of the heat supply to the evaporator (Fig. 2c).
Accordingly, these thermal storage processes are referred to as heat pumps.
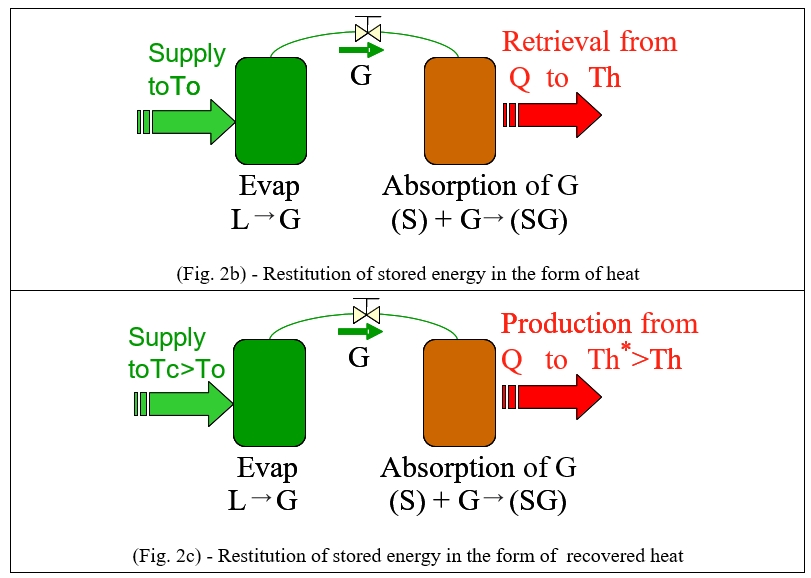
Figure 2: Thermochemical retrieval phase
Thermochemical storage has very significant capacities compared to traditional thermal storage units. These storage capacities depend largely on the nature of the physical or chemical bonds between the sorbent and the gas. Moreover, the existence of a wide variety of working sorbent/fluid couples covers a very wide temperature range (from -50 °C to 1300 °C).
In cold storage (Fig. 3), the thermochemical sorption processes allow remote cold production from available heat between 60 °C to 150 °C with a coefficient of performance ranging from 0.3 – 0.6. The use of ammonia or alcohols makes it possible to obtain negative cold systems up to -30 °C with storage capacities from 30 - 150 kWh.m-3. The use of water as a working fluid allows for much greater storage capacities from 50 - 300 kWh.m-3 but at positive temperatures suitable for solar cooling applications.
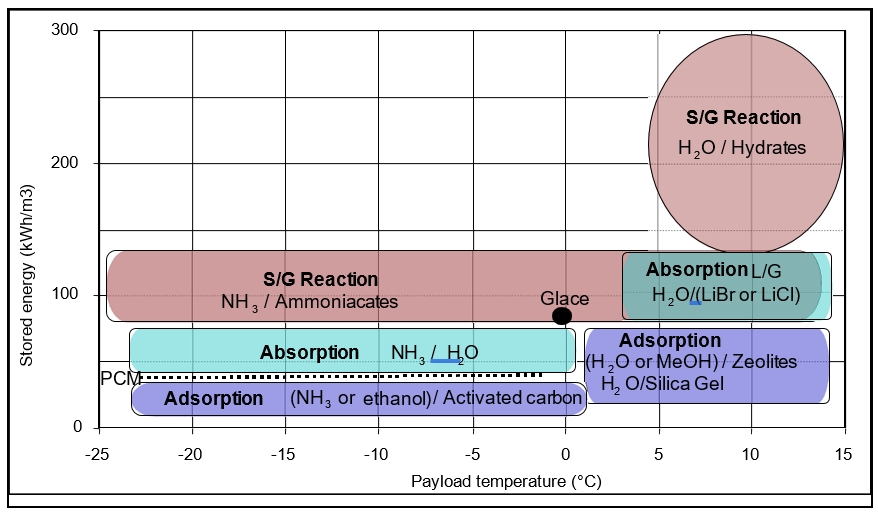
Figure 3: Cold storage capacity for different types of gas sorption processes
Application in temperature-controlled transport
The solid-gas sorption thermochemical process can be exploited in a meaningful way to produce remote cold, providing a temperature-controlled shipping container (roll container) with a significant cooling capacity. The principle is based on the liquid/gas phase change of a natural fluid (ammonia) and a reversible reaction between the latter and a solid reagent.
Insulated roll container
An insulated roll container equipped with a sorption system is a container made up of an insulated outer shell rotomoulded in food-grade polyethylene inside which polyurethane foam is injected. The overall coefficient of heat loss is 0.31 W/m-2.K-1. A roll container with external dimensions of 1200 x 800 x 2035 mm provides a payload size of 985 x 600 x 1310 mm.
Thermochemical gas-solid sorption unit
This roll container is equipped with a thermochemical solid-gas sorption unit, comprising of a thermochemical reactor and an evaporator connected to each other with a solenoid valve, both silently ventilated (figure 4). This device produces remote controlled cold which is regulated for a homogenous internal temperature. The production of cold is initiated when the user chooses, providing an effective cooling capacity to the container during transportation. The ammonia, salts and graphite are fully confined in a factory sealed circuit, which renders the system inaccessible and secure with no need for any filling. The roll container is equipped with an onboard tracking system making it possible to record the internal temperature of the container and detect when the door opens. The system provides an effective cooling capacity for 16 - 72 hours without being connected to the power grid (production cycle). It can be recharged on the power grid 230 V / 50-60 Hz in 6 hours 30 minutes (regeneration cycle). Figure 5 shows the operating principle of the thermochemical system.
The onboard energy corresponds to 7.8 kg of ammonia circulating between the evaporator and the thermochemical reactor, i.e. an energy of 2.8 kWh. The mean power on an 8 hour cycle is 350 W. The onboard energy varies according to the number of reactors integrated into the box. The number of thermochemical reactors is proportional to the amount of energy required. The range extends from 0.5 kWh – 8.4 kWh.
The theoretical COP for the thermochemical reaction is 0.5 because the enthalpy from regeneration is 2 times greater than the enthalpy from evaporation of the ammonia in cold production. The actual COP ranges between 0.25 and 0.5. Once the reactor is regenerated, the potential energy is stored “for free” until use, but this is not part of the COP calculation.

Figure 4: Roll container equipped with solid-gas sorption system
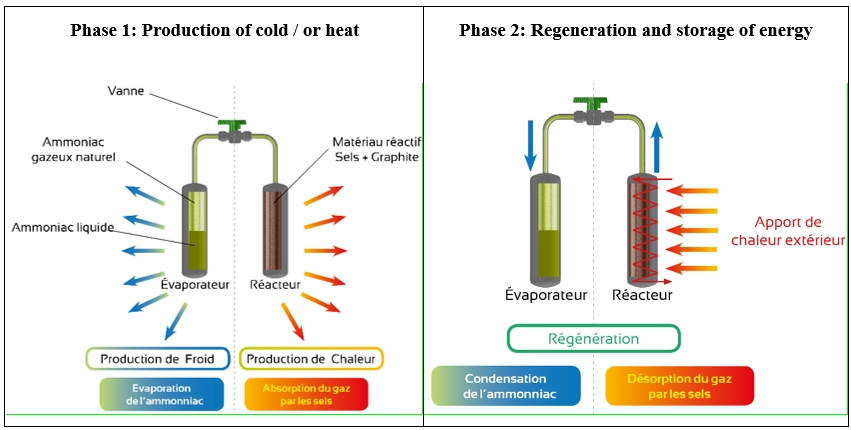
Figure 5: Operating principle of the thermochemical system
Thermal efficiency qualification tests
The roll container equipped with a solid-gas sorption system has been developed to store fresh products. We are interested in the qualification of the roll container for the shipping application of agri-food products between 0 and +4 °C under different external temperature profiles.
Test protocol
The shipping roll container was tested with 28 boxes with dimensions of 370 x 260 x 160 mm, including 6 loaded with 12 kg of tylose blocks, i.e. a total mass of 72 kg overall. 9 sensors (thermo-buttons) are placed on the product units, then the test load is stabilised at +2 °C for 48 hours. In order to cool the payload space in the container to +2 °C, the thermochemical module is switched on one hour before loading. Loading is performed at room temperature +22 °C, by placing the instrumented products in the upper and lower corners and in the middle of the sides of the container. In all tests, the setpoint is set at +2 °C, corresponding with the centre of the required storage range. The tests are performed in thermostatic chambers.
Tests under constant profiles +20 °C and +40 °C
The roll container was tested under constant external temperatures of +20 °C and +40 °C.
Below +20 °C (figure 6), the internal temperatures recorded by all of the sensors remained between 0 °C and +4 °C for at least 48 hours except for the sensor placed in the upper corner corresponding with an unfavourable position, which crossed the +4 °C limit after 35 hours.
Below +40 °C (figure 7), the internal temperatures recorded by all of the sensors remained between 0 °C and +4 °C for at least 24 hours except for the sensor placed in the upper corner which crossed the +4 °C limit after 16 hours.
The duration corresponds with the net cooling capacity after cooling the box from +21 °C to 0 °C, as well as the ammonia at its evaporation temperature (approximately -15°C). The cooling energy represents about 1/3 to 1/2 of the total energy. The cooling capacity depends on the temperatures of the box and the ammonia at the start of production and the temperature setpoint.
Figure 6: Cooling capacity of the roll container between 0 °C and +4 °C below +20 °C.
Figure 7: Cooling capacity of the roll container between 0 °C and +4 °C below +40 °C.
Test under the standard profiles ST-48-b and ST-48-d
The same roll container was tested under the standard profiles ST-48-b and ST-24-d using the NF S 99-700 standard.
Under the profile ST-48-b (figure 8), the internal temperatures recorded by all of the sensors remained between 0 °C and +4 °C for at least 48 hours except for the sensor placed in the upper corner which crossed the +4 °C limit after 45 hours.
Under the profile ST-48-d (figure 9), the internal temperatures recorded by all of the sensors remained between 0 °C and +4 °C for at least 48 hours. The curves show that the container can provide greater cooling capacity.
Figure 8: Cooling capacity of the roll container between 0 °C and +4 °C under the profile ST-48-b.
Figure 9: Cooling capacity of the roll container between 0 °C and +4 °C under the profile ST-48-d.
Test under a temperate profile
The same roll container was tested under a variable temperate profile consisting of segments alternated at +20 °C and +10 °C. The internal and external temperatures recorded are shown in figure 10. The internal and external temperatures recorded by all of the sensors remained between 0 °C and +4 °C for at least 72 hours. The container can provide more effective cooling capacity under this moderate profile.
Figure 10: Cooling capacity of the roll container between 0 °C and +4 °C under a temperate profile.
Conclusion
The thermochemical process based on solid-gas sorption stores thermal energy and produces cold remotely so that it can be used as needed. The temperature controlled use of this system with adjustable regulation, in insulated shipping containers secures the cold chain of perishable or heat-sensitive products with a cooling capacity ranging from 16 hours to 72 hours depending on external temperature conditions, the size of the reactors and the insulation of such containers.
These temperature-controlled containers combine the advantages of solutions equipped with eutectic plates or PCM and those with dynamic solutions equipped with refrigeration units, providing effective cooling capacity and temperature regulation. They can be easily shipped or integrated into standard vehicles. Use of a natural fluid reduces greenhouse gas emissions and the impact on the environment.
Authors: Abbes KACIMI, Cold Chain Expertise Manager, Sofrigam / Francis KINDBEITER, R&D Director, Coldway technologies, Driss STITOU, Thermodynamics, Energy and Reactive Systems (TES) Research Team Manager at CNRS-PROMES Laboratory