News
Thematic studies
Design of a labelled cooling pouch for the transport of medicines
The last stage of the distribution circuit, often managed by the patient, remains difficult to master; it is the weak link in the cold chain. What happens when the patient leaves the pharmacy with the vaccine to go home or to the doctor's office? How is he wearing the vaccine? Most importantly, did the product retain all the required qualities at the time of administration? These questions and especially the answers formulated by the users make it possible to carry out an initial risk analysis of this link, and to develop an adapted cold chain solution. Explanations.
Ensuring temperature maintenance throughout the supply chain
The various players in the drug supply chain (pharmaceutical laboratories, inspection and standards organisations, distributors, pharmacists, logisticians, solution providers, etc.) are stepping up their efforts to ensure that the cold chain is maintained throughout the supply chain, right up to the administration of the drug to the patient.
Many means are implemented to preserve the therapeutic qualities of the drug to be administered to the patient. Storage and transport conditions are relatively controlled from the manufacture of the product to its arrival at the pharmacy, via wholesaler-distributors.
But the distribution chain does not end there. Indeed, the last step before the administration of the product (pharmacy > home, home > dispensary...) is the weak link in the chain because it is often under the responsibility of the patient and out of the direct control of professionals.
So-called cooling pouches are used by patients to store heat-sensitive products between the pharmacy and the home or between the home and the doctor's office. A study conducted by Cemafroid* has shown that these pouches are not effective and are not suitable for storing heat-sensitive medicines between +2°C and +8°C.
The drug, a product with very low thermal inertia
The drug is a product with low thermal inertia, often less than 10% of that of water. Its temperature varies rapidly depending on the environment in which it is located.
A vaccine can go out of the required temperature range after a few minutes when unprotected and exposed to heat or cold. As a result of these variations, some products may degrade or lose their effectiveness.
Comparative test: the rise in temperature of a vaccine and a water pocket
A comparative temperature rise test is carried out on a vaccine and a small pocket of water. The vaccine is in the form of a glass vial containing 1.5g of liquid in a cardboard case. The plastic bag contains 25g of water. A probe that measures the temperature of the vaccine is placed on the glass vial inside the case. A probe is wound through the water pocket to measure the water temperature. The vaccine and the instrumented water bag are stabilized at +5 °C, and then placed in an environment at +21 °C (±1 °C).
The temperature of the vaccine rises rapidly, passing +8 °C after 3 minutes and reaching +15 °C after 12 minutes. The temperature of the water pocket crosses the +8 °C limit after 3 minutes and reaches +10 °C after 12 minutes.
The difference is explained by the difference between the thermal inertias of the two products. The result, shown on the curve below, shows the inevitable break in the cold chain if the vaccine is exposed unprotected to heat or cold.
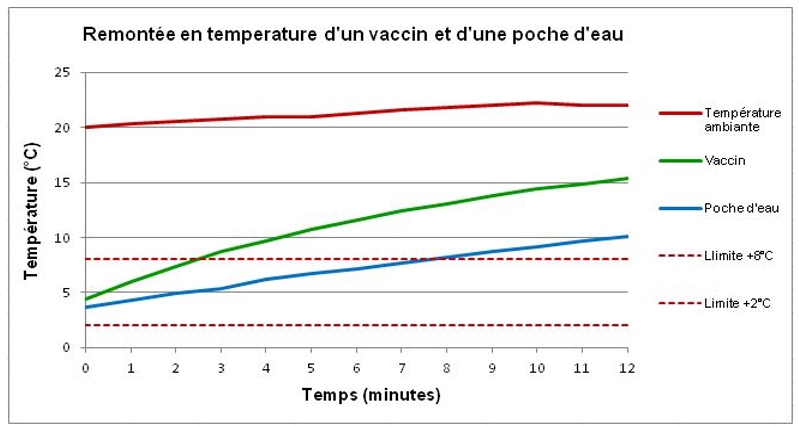
Temperature rise of a vaccine and a water bag to +21°C (±1°C)
Conventional cooling solutions do not meet the needs
The solutions usually proposed are so-called isothermal pouches, alone or combined with unsuitable eutectic plates. The arrangement of the products and the cold source varies according to the size of the products and the size of the pouch.
Having very thin walls and a relatively large exchange surface, the pouch alone without additional thermal inertia does not protect the product, whatever the quality of the insulating material. The use of an unsuitable eutectic plate does not significantly and reproducibly improve this performance. Such a plate covers only part of the product and may also deform the pouch and prevent it from closing.
According to the study carried out by Cemafroid, the conventional pouches used by patients are not effective. Tests have been carried out on different pouches to verify the holding time of a drug between +2 and +8°C under an outside temperature of +15°C.
- The duration do not exceed 5 minutes for pouches with thermal inertia and stabilizing the assembly at +5 °C.
- It does not exceed 3 minutes for pouches without thermal inertia stabilized at +5°C.
- The duration is negligible for pre-packaged pouches at room temperature.
Igloo° concept
Sofrigam has developed the Igloo° cooling pouch, designed for patients and hospitals to transport small products over short distances. It is a drug protection kit equipped with a flexible eutectic plate to be preconditioned at +5 °C. It is an efficient and easy to use single-configuration solution, with a single preparation method for all seasons. The Igloo° pouch keeps the product between +2 °C and +8 °C for at least 1 hour. Reusable at least 50 times, it secures the weakest link in the cold chain, from the pharmacy to the home, at a reduced price and within reach of the patient.
A set of specifications drawn up with pharmacists
A set of specifications was drawn up in collaboration with the actors in the drug distribution chain. Pharmacists and representatives of pharmaceutical companies, wholesale distributors, hospitals and pharmacies were consulted. The exchanges made it possible to take account of the constraints and requirements expressed and to compensate for the shortcomings and drawbacks of the existing insulated pouches. The solution sought is for patients and hospitals to transport small products over short distances. It must be effective, reusable and adapted to the means of pre-packaging available in pharmacies and at an affordable price. The main elements of the specifications are as follows:
- Maintain the product between +2 and +8 °C for at least 20 minutes, in summer and winter ;
- Homogeneity of temperature in the useful space occupied by the product ;
- Mono-configuration with the same simple operating mode and for all seasons ;
- Reusable and having reduced size and weight ;
- Acceptable price.
Description of the cooling pouch
The Igloo° kit is composed of an isothermal zip-closure pouch and a flexible plate of eutectic gel. The pouch is made by welding three plastic materials together. Two films sandwich a flexible polyurethane foam.
- The outer film is a complex of Corona-treated polyethylene terephthalate (PET), metallised Corona-treated PET and low density polyethylene (LDPE);
- The insulation is a flexible polyurethane foam;
- The inside is a white film of LDPE suitable for food contact.
- The flexible eutectic plate is composed of 6 doses of gel packaged in plastic film bags.
Instructions for use are printed on the pouch.
The independent Certicold marking is affixed to the pouch.
The external dimensions of the pouch are 260 x 200 x 25 mm for a total weight with the eutectic plate of 277 g.
A qualified and certified cooling solution
Igloo° has been the subject of a qualification of thermal performances by the laboratory Ater Métrologie and of a Certicold Pharma certification by Cemafroid (certificate number: CTCPE-FR-002-002). It keeps the product between +2 and +8 °C for at least 1 hour. Test results are reproducible and confirmed by the certifying laboratory. Two patents have been filed for the concept and for the brand.
Conclusion
The Igloo° cooling pouch is designed for patients and hospitals to transport small products over short distances. It is a drug protection kit equipped with a flexible eutectic plate to be preconditioned at +5°C. It is an efficient and easy to use single-configuration solution, with a single preparation method for all seasons. The Igloo pouch keeps the product between +2 °C and +8 °C for at least 1 hour. Reusable at least 50 times, it secures the weakest link in the cold chain, from the pharmacy to the home, at a reduced price and within reach of the patient.


